
Advancing Incontinence Care

Advancing Incontinence Care
Imaging & Procedural Compatibility
Prior to Axonics' launch in 2019, Sacral Neuromodulation (SNM) patients had limited access to imaging and diagnostic procedures. Today, SNM patients have the best MRI conditions for active implantables among the entire medical device industry.
Broad MRI Conditional Labeling
As of February 2025 we have expanded labeling that includes 3T full-body coil with open impedances and fragments of Axonics or Medtronic leads. This updated labeling is retroactive to all existing Axonics implants.


Visit www.axonics.com/resource-library to download full MRI Guidelines
Axonics is driving Therapy Access beyond just MRI. Low rates of impedance issues minimize the disruption to therapy, and enhanced SNM system compatibility for other procedures, treatments, or activities provides easier access.
Axonics designed our tined lead to be durable so that impedance issues are minimized. In contrast, legacy InterStim® tined leads have shown a high rate of impedance issues.1
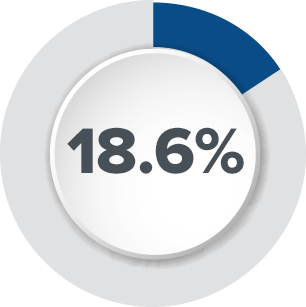
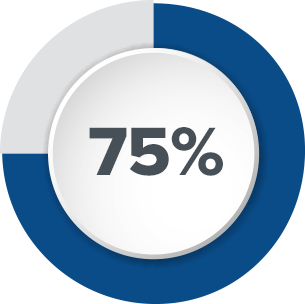



Based on feedback from physicians and patients, Axonics has invested in research and testing to demonstrate safety and compatibility with common procedures and activities. Enhanced labeling makes it easier for patients to get the procedures they need.
Labeling Comparison for Common Procedures
 RF Ablation
RF Ablation Lithotripsy (shockwaves of kidney)
Lithotripsy (shockwaves of kidney) High Intensity Focused Ultrasound (HIFU)
High Intensity Focused Ultrasound (HIFU) Physiotherapy Ultrasound
Physiotherapy Ultrasound Pelvic Floor Muscle Stimulator (e.g., Emsella Chair)
Pelvic Floor Muscle Stimulator (e.g., Emsella Chair)
Labeling Comparison for Active Patients
For more information about all compatible medical procedures, visit www.axonics.com/resource-library to download our latest 'Information for Prescribers and Patients' manual.

When it comes to choosing an SNM System, reliable performance, patient experience and support make a difference. Axonics SNM Systems include innovations to address the shortcomings of legacy devices and improve the patient experience.
References:
InterStim is a registered trademark of Medtronic, Inc.